News
| 2024/4/1 | Notice Associated Professor Takumi Ueda was promoted to the professor in Graduate School of Pharmaceutical Sciences, Osaka University. Congratulations! | |||||||||||||||||||||||||||||||
| 2023/11/9 | Award Assistant Professor Yutaka Kofuku awarded the Young Scientist Award (Shinposho) from Nuclear Magnetic Resonance Society of Japan.Assistant Professor Yutaka Kofuku PhD. was awarded the prise for his substential contribution to the advance of science by using NMR. | |||||||||||||||||||||||||||||||
| 2023/5/22 | Publication A paper showing that subterahertz irradiation accelerate the microscopic mixing of water and proteins was published.Comprehensive analysis using sub-THz wave irradiation, microwave-band dielectric measurements, NMR spectroscopy, and terahertz spectroscopy has revealed that sub-THz wave irradiation of a protein solution excites the movement of water molecules around the protein, resulting in a change in hydration state. | |||||||||||||||||||||||||||||||
| 2022/11/10 | Award Associate Professor Takumi Ueda awarded the Young Scientist Award (Shinposho) from Nuclear Magnetic Resonance Society of Japan.Associate Professor Takumi Ueda PhD. was awarded the prise for his substential contribution to the advance of science by using NMR. | |||||||||||||||||||||||||||||||
| 2022/10/24 | Notice Prof. Kurt Wüthrich, 2002 Nobel Prize Laureate in Chemistry, gave us a lecture on "Life with NMR". |  | ||||||||||||||||||||||||||||||
| 2022/7/1 | Publication The Structure paper was accepted to the cover. |  | ||||||||||||||||||||||||||||||
| 2022/5/3 | Publication Unraveling the Trick of Human GTP Sensor Protein Evolution -Evolutionary Acquisition of PI5P4Kβ for Stress Resilience-Structure, 2022, 30(6), P886-899.E4
A joint research team of the University of Tokyo, University of Cincinnati, High Energy Accelerator Research Organization (KEK), Keio University, Rikkyo University, Hoshi University, Tokai University, and Kansai Medical University unveiled a way that a GTP-sensing kinase PI5P4Kβ evolutionary acquired a function to sense GTP levels in cells and regulate energy metabolism and cell growth. Figure: Recognition of ATP and GTP by GEA motifs and their evolutionary acquisition. | 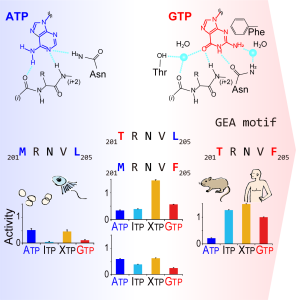 | ||||||||||||||||||||||||||||||
| 2022/5/2 | Notice The 4th Workshop on NMR from the Basics - NMR in Drug Discovery and Industrial Research -Dates: Thursday, May 19 ~ Friday, May 20, 2022 In drug discovery, a comprehensive evaluation of the structures, interactions, and dynamics of drug molecules and their target biomolecules is necessary. In such situations, nuclear magnetic resonance (NMR), which provides multifaceted information about molecules in solution, is used as an effective drug discovery research tool. In addition to drug discovery, NMR can also provide useful information in various situations, especially when various physical properties are present, such as in the study of foods and chemical products. This workshop will focus on NMR and structural analysis techniques in drug discovery and industrial research. Active researchers from academia and industry will give lectures aiming to exchange information between industrial and academic research. Program:
5/20
Organized by: Institute for Protein Research, Osaka University Pre-registration and more information: https://nextnmr.jp |  | ||||||||||||||||||||||||||||||
| 2022/3/28 | Notice The new webpage of the Laboratory of Physical Chemistry was launched. |  | ||||||||||||||||||||||||||||||
| 2022/3/1 | Notice Defense presentation for the doctoral thesis of Mr. Tomoki Yokomizo, and those for master thesis of Ms. Aya Aoki and Mr. Katumune Donai were successfully conducted. Everyone conveyed fanatic presentations. |  |
